
Welcome to episode 441 of The Whole View! This week, Stacy and Sarah break down the science behind the Pfizer/BioNTech and Moderna vaccines and the safety standards of each.
If you enjoy the show, please review it on iTunes!
The Whole View, Episode 441: COVID-19 Vaccines Part 2 – Pfizer/BioNTech vs Moderna
Welcome back to episode 441 of the Whole View. (0:27)
Stacy reminds everyone that this is part 2 of the Covid show on vaccines.
So if you’ve done listened to part 1, be sure to do so first, so this episode will make more sense.
She also reminds listeners that this show isn’t about opinions. We all have our own, and Stacy and Sarah aren’t here to debate that.
As always, they are here to break down the science for you to make your own informed choices.
Stacy takes a moment to thank Sarah for doing all the research to make these shows possible.
She and Sarah received a lot of feedback from listeners who enjoyed a straightforward approach to this topic.
Sarah explains that it’s always more challenging when she knows they’re getting into a topic where there’s a lot more disinformation and misinformation to combat.
Especially when they take a more myth-busting approach because that information could have come to you from a trusted source.
Sarah extends her gratitude to listeners for being so open to the science and having an open mind.
Sarah takes a minute to run through a recap of mRNA vaccines and how they work, which she covered extensively in Episode 440.
She also reiterates how important funding for basic science discovery is because we almost didn’t get this mRNA research.
Different Variants of Concern
Sarah reminds listeners that the variations we see in the virus are different enough to count as a new strain. (15:50)
They’re called “variants of concern” because they have a few mutations that change the virology (basically make a function change).
We know the novel coronavirus mutates very slowly. But because of the sheer number of people, it’s infected so far this year, it’s had many opportunities to mutate.
The UK and South African variants have a mutation in common, which is how easily the spike protein binds to the ACE2 receptions and speeding up the replication rate.
This means the infection dose we need to be exposed to become infected is slower. It also means an infected person is shedding more virus than other strains.
In fact, the UK strain is showing 40%-80% more contagious than the original virus.
Pfizer/BioNTech and Moderna both have looked into how the antibodies bind to the different strains, including the UK and South African variants.
Why Target The Spike Protein?
Antibodies we make after vaccination bind with the spike protein to block ACE2 binding.
Pfizer/BioNTech has shown that the antibodies that study participants made in response to vaccination effectively bind to and neutralize the UK strain.
Moderna has shown its vaccine is equally good at neutralizing the UK strain.
The vaccines may not work as well against the South African strain but will still provide some protection.
Early data using convalescent plasma shows antibodies against other covid-19 strains don’t effectively neutralize the South African strain.
That’s bad news for monoclonal antibody treatments, convalescent plasma treatments, and natural infection.
While the Company expects these levels of neutralizing antibodies to be protective, pseudovirus neutralizing antibody titers were approximately 6-fold lower than prior variants.
These lower titers may suggest a potential risk of the earlier waning of immunity to the new B.1.351 strains.
Moderna is working on additional boosters to help bind the South African variant. For more information, check out their latest new release.
Good news, though- a second vaccine against this strain would be easy to make!
Both UK and South African variants have N501Y mutation, which confers a replicative advantage, so when you start shedding the virus, you shed more of it.
This is how scientists currently believe it’s more contagious.
The South African variant also attaches to ACE2 receptors more strongly because of two other mutations, E484K and K417N.
Almost all vaccines require “boosters,” which is why the Covid vaccine requires two doses to be effective.
- the first dose primes the immune system
- the second dose elicits even more of a response for a more robust immunological memory
Myth: The Vaccines Were Rushed
The Pfizer/BioNTech and Moderna vaccines’ development broke records as the fastest vaccine ever produced. (24:01)
Sarah reiterates from last week’s episode that this was made possible by recent mRNA vaccine technology breakthroughs.
Not because any corners were cut!
The speed is also attributed to having the funds necessary to do multiple things when normally these steps would have been done one at a time.
Because we give vaccines to healthy people, the standard of safety is much higher!
Safety & Efficacy: Phase 2/3 Clinical Trial Data
Sarah explains that phase 2 clinical trials test safety and phase 3 tests for efficacy. (30:05)
It is possible to do phases simultaneously, which happened with the Pfizer/BioNTech and Moderna vaccines and why it’s called Phase 2/3.
All the research has since gone through extensive review, both peer and independent, to provide recommendations to the FDA.
Sarah wants to emphasize just how many expert eyes have been on this project. And that this isn’t something to be messed up.
It’s also important to note that safety and efficacy are ongoing even after distribution.
Preventing Infection:
Pfizer/BioNTech:
- A total of 43,548 participants underwent randomization of whom 43,448 received injections: 21,720 with BNT162b2 and 21,728 with placebo.
- There were 8 cases of Covid-19 with onset at least 7 days after the second dose among participants assigned to receive BNT162b2 and 162 cases among those assigned to placebo;
- BNT162b2 was 95.3% effective in preventing Covid-19 (95% credible interval, 90.3 to 97.6).
Moderna:
- The trial enrolled 30,420 volunteers randomly assigned in a 1:1 ratio to receive either vaccine or placebo (15,210 participants in each group).
- More than 96% of participants received both injections, and 2.2% had evidence (serologic, virologic, or both) of SARS-CoV-2 infection at baseline.
- Symptomatic Covid-19 illness was confirmed in 185 participants in the placebo group (56.5 per 1000 person-years; 95% confidence interval [CI], 48.7 to 65.3) and in 11 participants in the mRNA-1273 group (3.3 per 1000 person-years; 95% CI, 1.7 to 6.0)
- The vaccine efficacy was 94.1% (95% CI, 89.3 to 96.8%; P<0.001).
Preventing Severe Disease:
Pfizer/BioNTech
- Among 10 cases of severe Covid-19 with onset after the first dose, 9 occurred in placebo recipients and 1 in a BNT162b2 recipient.
- 90% effective at preventing severe covid-19
Moderna
- Severe Covid-19 occurred in 30 participants, with one fatality
- All 30 fatalities were in the placebo group
- 100% effective at preventing severe covid-19
Sarah explains that there is a lot of overlap in effectiveness between the Pfizer/BioNTech and Moderna vaccines, which means they’re basically identical.
Both she and Stacy tell listeners that they are good with taking whichever is available to them first.
Immune Reaction:
Sarah reminds listeners that immunity after natural infection is actually pretty low. (34:45)
We’re still not sure how long immunity from Covid-19 lasts. We know that after 4 months, we still have a lot of protection after vaccination, and we are still monitoring this as time progresses.
At 4 months, results show antibodies are higher after vaccine than in cases of natural infection.
Immunological memory for coronaviruses is quite low: 1 year for the common cold up to 5 or 6 years for SARS.
This is partly because coronaviruses manipulate our immune systems to evade detection, most notably interfering with interferon production and antigen presentation.
We develop an immune response with vaccination without the immune system manipulation from the virus as natural infection.
We still may need regular boosters.
But early evidence shows that vaccine immunity will be longer lasting than natural immunity!
What If You’ve Already Had Covid?
Most patients develop antibodies after they have COVID-19, but not all patients do.
In other coronavirus infections (such as the common cold), we know that these antibodies last only three to four months before we are susceptible to infection again.
The vaccine produces a strong immune response and lasts at least four months. Check here for more information!
Volunteers who received the Moderna shot had more antibodies in their blood than those who had been sick with Covid-19.
The Centers for Disease Control and Prevention recommends that patients who recently had COVID-19 should wait 90 days before receiving the vaccine.
It’s recommended to wait 90 days after recovering before getting the vaccine.
But because results show better and longer-lasting immunity, vaccination is still beneficial to those who have already had it.
Subgroup Analysis: Pre-Existing Conditions
Test groups with pre-existing conditions were not exceeded from phase 2 clinical testing. (40:45)
However, autoimmune diseases aren’t concerned with the at-risk group for Covid-19, except if you’re on heavy immunosuppressants.
Because people with autoimmune diseases aren’t considered at-risk, there isn’t a specific subgroup analyzing them.
Instead, a subgroup looked at age, sex, race, ethnicity, baseline body-mass index, and the presence of coexisting conditions.
Pfizer/BioNTech shows similar vaccine efficacy (generally 90 to 100%) across subgroups defined by age, sex, race, ethnicity, baseline body-mass index, and the presence of coexisting conditions.
Moderna efficacy was similar across key secondary analyses, including assessment 14 days after the first dose, analyses that included participants who had evidence of SARS-CoV-2 infection at baseline, and analyses in participants 65 years of age or older (range 86.4% to 100%)
Patients who are immunocompromised (HIV/AIDS, cancer therapy, immunosuppressant drugs – not autoimmune disease) were not included in the initial studies.
We do know that patients with underlying conditions are at higher risk of severe COVID-19 disease.
The CDC recommends that these individuals still receive the COVID-19 vaccine unless there is a contraindication (such as an allergy to prior vaccines).
In fact, Moderna included HIV, 0/80 in vaccination, 1/76 in placebo = 100%
Sarah reminds listeners that even though the vaccine does offer individual protection, that’s not what vaccination is all about. It’s about community protection!
What We Don’t Know
Stacy adds that just because you have the vaccine doesn’t mean you can go about your daily life now like you did pre-pandemic.
This is because many others in your community might not be vaccinated yet.
And while you’re protected, you could still be shedding virus onto those who have not had the opportunity to.
Combining the vaccine with social distancing and wearing masks can put us in a position to make a difference.
We don’t know how long immunity will last and if or when we’d need a booster for it.
Based on what we know of other coronaviruses, experts expect to need a booster every 1-5 years. But we just don’t know yet.
We also don’t know if it’s possible to get an asymptomatic case of Covid-19 after being vaccinated. In fact, it may even be more likely.
That’s not all bad, as symptomatic infections are the ones that lead to fatal medical complications.
However, it does increase the risk of spreading the virus to those who have not been vaccinated.
For example, if they are medically not allowed due to cancer or other illness.
Because asymptomatic cases won’t help stop the spread until much later in vaccination, that’s why we’re being asked to continue pandemic protocol even after receiving the vaccine.
Safety
Sarah explains that everything you’d possibly want to track was tracked for the Pfizer/BioNTech and Moderna vaccines’ clinical trials.
For Pfizer/BioNTech, the safety profile of BNT162b2 was characterized by short-term, mild-to-moderate pain at the injection site, fatigue, and headache.
The incidence of serious adverse events was low and was similar in the vaccine and placebo groups.
In Moderna, serious adverse events were rare, and the incidence was similar in the two groups.
There were no specific safety concerns identified in subgroup analyses by age, race, ethnicity, medical comorbidities, or prior SARS-CoV-2 infection.
The most common reactions were injection site reactions (84.1 percent), fatigue (62.9 percent), headache (55.1 percent), muscle pain (38.3 percent), chills (31.9 percent), joint pain (23.6 percent), and fever (14.2 percent).
These are short-lived and similar to what we see with influenza or shingles vaccines.
Remember, these side effects mean that your immune system responds to the vaccine and creates antibodies against COVID-19!
So these symptoms are telling you your immune system is working.
When it comes to boosters and countries rolling out their vaccines, Sarah explains that it’s a situation where there’s not always a right answer.
Severe Reactions
Sarah explained in depth why severe reactions happen in part 1 (55:10).
However, severe reactions for the Pfizer/BioNTech and Moderna vaccines are rare in clinical trials due to the smaller number of test subjects.
If there is an instance of severe reaction during clinical phases, the trials would be paused.
Sarah goes over what some of severe adverse reactions and some of the rare reactions that we know other vaccines can cause, such as PEG allergy, Encephalitis, Thrombocytopenia, Gillian-Barre syndrome.
There have been instances of allergic reactions with the Pfizer/BioNTech and Moderna vaccines.
So if you’re someone who carries an EpiPen for various anaphylaxis allergies, your doctor may advise you to hold off on getting the vaccine.
This is because they’re still trying to figure out what is causing the reaction.
If you know you’re at risk, it’s safer to hold off until they isolate what’s causing it and whether or not you’ll react to it.
Sarah goes on to say that the odds of a severe reaction are very, very low.
For example, in the US, there were 6 cases of anaphylaxis reported in the first 272,000 vaccinations.
She says this is why the protocol is that you sit there and wait once you get the vaccine. That way, if you react, you can get quick medical care.
Sarah also explains that that 1 in 50,000 hasn’t held since that first wave of testing. Now we’re talking more 1 in a 1/4 or 1/2 million, which is a very low frequency.
Pfizer/BioNTech 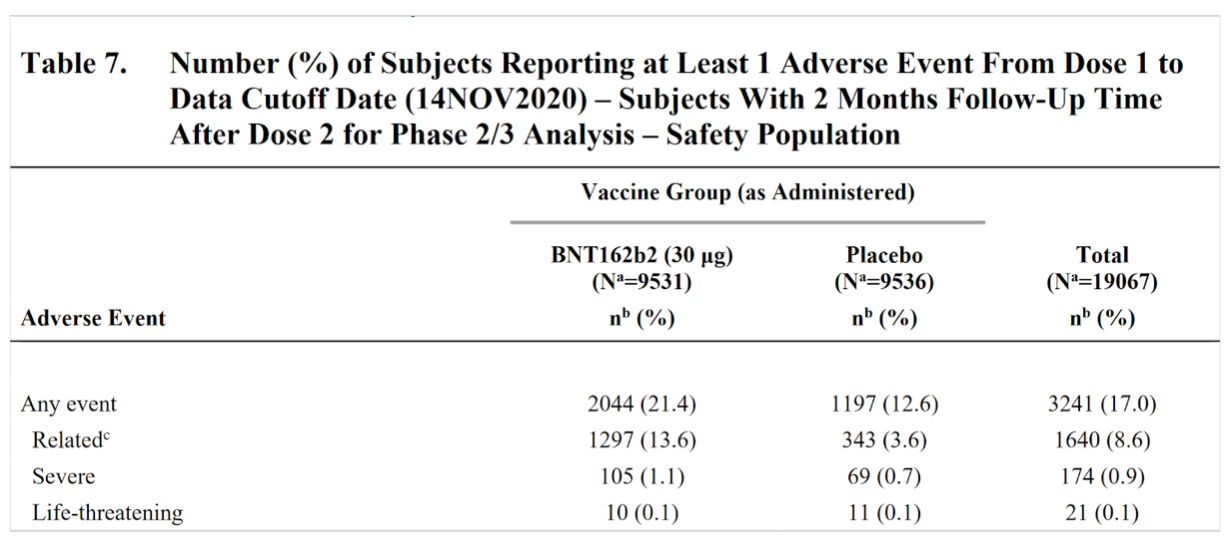 Moderna
Moderna 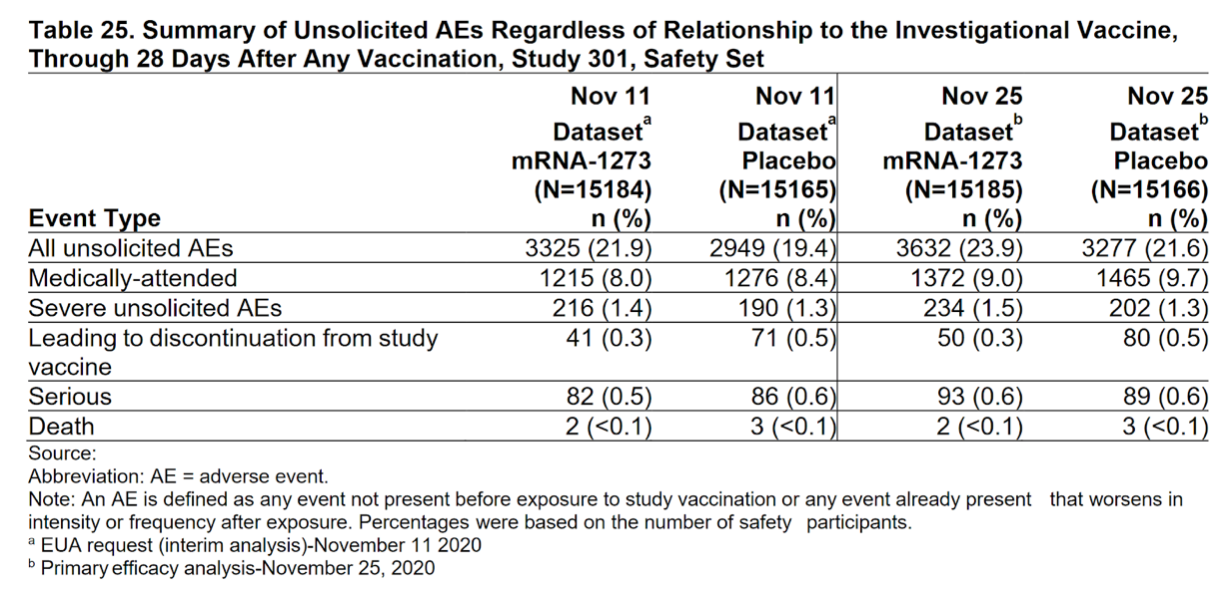
Final Thoughts
Stacy reminds listeners that education and information are how you make informed indecision.
So in talking about adverse reactions, it’s not meant to scare you but give you all the information available to help you choose what’s best for you.
She and Sarah were a bit looser this week with their viewpoint of getting the vaccine.
They acknowledge they’re human and have their own bias.
But Stacy hopes in by bringing all this information to you, you can feel safe and comfortable with whatever decision you decide.
She and Sarah are not medical professionals and are not here to tell you what to do.
For Stacy, her decision to get the vaccine is based on her health and best for her personally.
Her mother, who has anaphylaxis, would have to make her own choice with her own doctors for what’s best for her.
So Stacy feels it’s incredibly important to clarify that you should do what’s best for you and your health and not make a decision based on what she and Sarah are doing.
Sarah and Stacy want to center this entire conversation on the scientific evidence, data, and facts and not enter it from a position of fear, anxiety, judgment, or emotional bias that is often brought into these types of topics.
If you have follow-up questions or myths you’d like busted or confirm, submit questions via social media pages, websites, newsletters, or on Patreon.
In fact, joining Patreon is the best way to get ahold of Stacy and Sarah.
Be sure to pop over there for bonus content and to hear what Stacy and Sarah really feel!
Thank you so much for listening. We will see you next week!





