In December of 2022, through the legislative process that approves the budget, the US Federal Government passed the first ever substantial health protective cosmetics-related amendment to the Food, Drug, and Cosmetic Act (FDCA) since its enactment in 1938. The problem has been, from both industry pushback and political push back, nothing has been implemented (yet). Finally, on March 8th, 2024 HR 4366 delivered FDA Funding for MoCRA.
The Modernization of Cosmetics Regulation Act of 2022 (MoCRA) creates a comprehensive regulatory framework that imposes new FDA registration and listing requirements, labeling rules, enforcement authority, and good manufacturing practices (GMP) requirements, among other regulatory obligations, on cosmetic manufacturers. I wrote more about that here (1) and FDA provides a summary here (2).
Let’s look at what this new funding means for us as consumers.
First-Ever FDA Funding for MoCRA Implementation
Beautycounter positively shared:
the Consolidated Appropriations Act of 2024, a bipartisan bill that includes $7 million in funding for the FDA to support the implementation of MoCRA, was signed into law.
As a partner with Beautycounter, I have advocated for Federal support and proper implementation of cosmetics regulatory reforms. And while we are energized to continue to fight for more policies that still need to be, I’m wondering what $7 million going to do towards the goals of MoCRA.

2023 MoCRA Funding
Although FDA was told to plan the implementation of MoCRA for the FY 2023-24, a report from GAO a year later concluded the FDA still has not addressed some components of implementing the various MoCRA reforms and managing the workforce. This includes, for example, developing a written implementation plan for carrying out MoCRA requirements, reporting on its own progress in implementing MoCRA, strengthening DEIA in its hiring and subsequent implementation of the law, identifying all personnel needed to implement MoCRA, drafting position descriptions for new hires, and creating a recruitment system.
Not surprising, since the FDA wasn’t given any funding when MoCRA was enacted.
The FDA had an opportunity to comment on GAO report, and generally agreed with GAO’s findings and recommendations. Although MoCRA authorized FDA funding levels that increase sharply between 2023 to 2027, no funding has been specifically set aside for MoCRA implementation. FDA has since requested additional funding for 2024 in order to hire new staff to assist in implementing MoCRA, and the agency anticipates requesting funding for additional staff going forward in order to fully implement all of MoCRA’s provisions. (6)
Consolidated Appropriations Act, 2024
Which brings us to the first funding for MoCRA ever, in March 2024 (15 months later). The FDA requested $72 Billion (B, not M) to fund their needs. From infrastructure upgrades to funding for workforce expansion, their funding proposal is extensive. The FY 2025 request, which covers the period from Oct. 1, 2024, through Sept. 30, 2025, includes new efforts for their high-priority program areas.
Highlights of the agency’s request include:
$8 million in additional funds to support the implementation of the Modernization of Cosmetics Regulation Act (MoCRA). The FDA will use the funding increase to further develop a modernized cosmetics regulatory program and enhance the agency’s efforts to protect consumers and help ensure the safety of cosmetic products. Funds will be used for activities such as developing regulations and compliance policies; managing submission platforms associated with MoCRA provisions; reviewing MoCRA-required information submitted to the FDA for industry compliance; and hiring additional subject matter experts to manage critical projects, such as the assessments of the use of perfluoroalkyl and polyfluoroalkyl substances (PFAS) in cosmetic products. (4)
The legislation, which is included at Section 3501 of the Food and Drug Omnibus Reform Act of 2022 (FDORA, 9) as part of the Consolidated Appropriations Act, 2023 (all of which means, was slipped in through a big funding package vs. passing separate laws), finally brings the FDA’s authority over the beauty and personal care sector more in line with what most consumers already believed and similar to other categories, such as drugs, devices and foods, the agency oversees. It requires cosmetic manufacturers to register their facilities and list their products, thereby putting such entities and products on the FDA’s radar. The FDA also increases its oversight of cosmetic safety by requiring adverse event reporting, GMP and record keeping requirements. The act takes effect on Dec. 29, 2023 (which is actually FY24 because fiscal years start in September the year prior). (8)

A summary of HR 4366 is as follows:
This bill provides FY2024 (October 1, 2023, through Sept. 30, 2025*) appropriations for several federal departments and agencies. It also extends several expiring programs and authorities, including several public health programs.
*yes, the Government is funding itself iteratively in arrears. this process funds its own multi-billion dollar service industry to work on procurements – and was my career speciality (5).
Specifically, those funded in the bill include:
- Department of Defense military construction and family housing activities,
- Department of Agriculture,
- Food and Drug Administration,
- Department of Commerce,
- Department of Justice,
- the science agencies,
- Department of Veterans Affairs,
- Department of Energy,
- U.S. Army Corps of Engineers,
- Department of the Interior,
- Environmental Protection Agency,
- Department of Transportation,
- Department of Housing and Urban Development, and
- several related and independent agencies.
In addition, the bill include several provisions that extend or otherwise modify various programs and authorities. These include several public health programs.
The bill also modifies the Compacts of Free Association that govern the relationship between the United States and the Republic of the Marshall Islands, the Federated States of Micronesia, and the Republic of Palau.

Where does the Money Go?
TITLE IV: Department Of Health And Human Services, Food & Drug Administration Salaries and Expenses
For necessary expenses of the Food and Drug Administration, including … that of the total amount appropriated:
(1) $1,185,989,000 shall be for the Center for Food Safety and Applied Nutrition and related field activities in the Office of Regulatory Affairs;
(2) $2,334,704,000 shall be for the Center for Drug Evaluation and Research and related field activities in the Office of Regulatory Affairs;
(3) $570,632,000 shall be for the Center for Biologics Evaluation and Research and for related field activities in the Office of Regulatory Affairs;
(4) $284,285,000 shall be for the Center for Veterinary Medicine and for related field activities in the Office of Regulatory Affairs;
(5) $770,697,000 shall be for the Center for Devices and Radiological Health and for related field activities in the Office of Regulatory Affairs;
(6) $77,505,000 shall be for the National Center for Toxicological Research;
(7) $684,324,000 shall be for the Center for Tobacco Products and for related field activities in the Office of Regulatory Affairs;
(8) $215,701,000 shall be for Rent and Related activities;
(9) $230,423,000 shall be for payments to the General Services Administration for rent; and
(10) $367,522,000 shall be for other activities, including the Office of the Commissioner of Food and Drugs, the Office of Food Policy and Response, the Office of Operations, the Office of the Chief Scientist, and central services for these offices:
Provided further, That any transfer of funds pursuant to, and for the administration of, section 770(n) of the Federal Food, Drug, and Cosmetic Act (21 U.S.C. 379dd(n)) shall only be from amounts made available under this heading for other activities and shall not exceed $2,000,000
User Fees
Note: this was a shock to me so I’ll share while we’re on the topic. The FDA (like USPS) actually makes money for itself, a rarity for public agencies. Those fees were:
- $362,381,000 derives from medical device user fees
- $613,538,000 derives from human generic drug user fees
- $31,109,000 derives from biosimilar biological product user fees
- $33,500,000 derives from animal drug user fees
- $25,000,000 derives from generic new animal drug user fees
- $712,000,000 derives from tobacco product user fees
Not including mammography user fees export certification user fees, priority review user fees, food and feed recall fees, food reinspection fees, and voluntary qualified importer program fees, outsourcing facility fees, prescription drug wholesale distributor licensing and inspection fees, third-party logistics provider licensing and inspection fees, third-party auditor fees, medical countermeasure priority review voucher user fees, and fees relating to over-the-counter monograph drugs (no values for which were specified).
That’s $1,777,528,000 – $1.8 Billion; making the funding for health protection on MoCRA 00.39% – one third of one percent.
What the FDA Listed for Funding for MoCRA
Implementation of MoCRA isn’t listed in either the FY24 House Bill or the FY25 request by name. In fact, MoCRA and the Food and Drug Omnibus Reform Act of 2022 aren’t even listed as a related bill to the funding for this year (that whole $2M).
BAD NEWS:
While Congress intended funding to “ramp up” and be distributed to the FDA to implement MoCRA, the only reference we can see in this FY24 funding document is for “Federal Food, Drug, and Cosmetic Act shall only be from amounts made available under this heading for other activities and shall not exceed $2,000,000.”
And even worse… further added:
Sec. 722. None of the funds made available by this Act may be used to propose, promulgate, or implement any rule, or take any other action with respect to… Federal Food, Drug, and Cosmetic Act, unless and until a Federal law is enacted to allow or require such distribution. (3)
If I worked for a consulting company assisting the FDA implementation of MoCRA, I’d walk away. It appears that MOCRA lacks funding in order to hold up implementation.
GOOD NEWS:
To complement the funding requests, the agency’s budget proposal also includes a package of legislative proposals designed to better support agency efforts to protect American consumers and patients. Including some which would apply to personal care and supplement industries you hear me often reference:
- Provide new authorities to help ensure the safety of foods. This includes new authority to set binding contaminant limits by administrative order, requirements for contaminant testing of final products, more frequent environmental monitoring for pathogens in certain facilities, and mandatory reporting when certain products test positive for pathogens.
- Provide additional oversight tools. This includes items such as expanding authorities for information-sharing with states, broadening authority to request records or other information in advance of or in lieu of inspections to all FDA-regulated commodities, and requiring importers to destroy products that present a significant public health concern.
- Propose new authorities that would require animal drug sponsors to make post-approval safety changes and expand the FDA’s mandatory recall authority to cover all human and animal drugs.
- Provide the FDA with additional authorities to increase oversight of dietary supplements to better protect consumers from dangerous and otherwise illegal products on the market.
These are some incredibly hopeful requests that would go a long way to protect Americans. I personally contacted my representatives to tell them to support the FDA’s 2025 funding and proposed legislation. You can read more in their FY25 Legislative Proposal. (7)
MY TAKE:
The industry pushback on implementing MoCRA (which literally pushed back the implementation date to July 2024) (10), has driven MoCRA out of bipartisan support into political fodder. It’s hard to not feel like the funding allocation, timing, and consistent delays aren’t negatively impacting people and planet.
If the GAO report can motivate Congress to fund the FDA’s FY25 request, it will show a shift in a positive direction. Sadly my bet is that we continue to have iterative minimal funding (across all Federal government) without allowing Agencies to strategically support the people and intention of the laws previously passed.
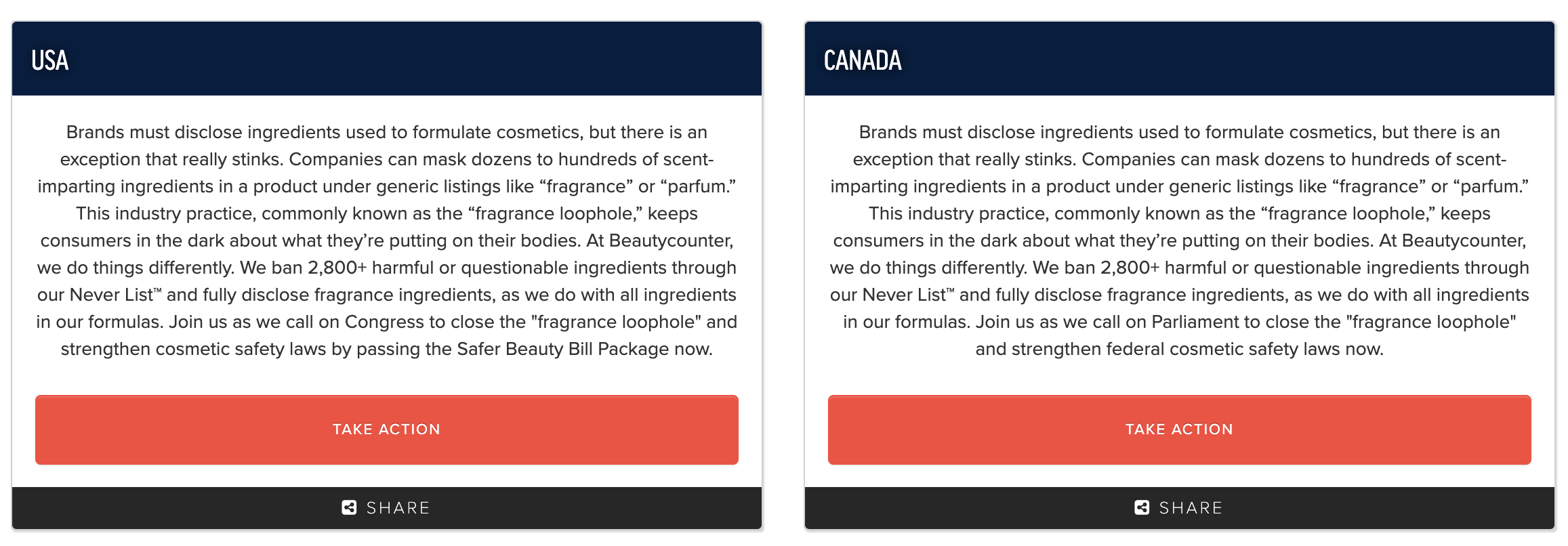
Reminder: even when the FDA Funding for MoCRA comes fully implemented, it doesn’t touch closing the fragrance loophole, banning the worst chemicals, increasing supply chain transparency, or protecting overexposed communities.
We as a community much take action.
If you’re not sure how to go about contacting your representatives, you can simply Google who they are and call the office (the easiest that is the most effective). Or click below and edit the form letter drafted to your specific reps tailored to your thoughts. Remember: the more personal the better.
Sources:
- RealEverything’s First Federal Law Update to Personal Care Since 1938: All About the Modernization of Cosmetics Regulation Act
- FDA.gov, Modernization of Cosmetics Regulation Act of 2022 (MoCRA)
- H.R. 4366; Consolidated Appropriations Act, 2024
- FDA.gov, FDA Seeks $7.2 Billion to Enhance Food Safety and Nutrition, Advance Medical Product Safety, and Strengthen Public Health
- GSA.gov, Federal Acquisition Service top impacts in fiscal year 2023
- Cromwell.com, GAO Report Concludes that the FDA Needs to Strengthen its Efforts to Implement MoCRA
- FDA.gov, FDA 2025 Legislative Proposals
- Loeb & Loeb LLP, MoCRA Increases FDA Oversight of the Cosmetics Industry
- H.R. 2617; FDORA
- The National Law Review, MoCRA Update for Starting the Year Off Right