What is the Modernization of Cosmetics Regulation Act (MoCRA)? It’s the first Federal law to update personal care products in any way since 1938. You know, when hundreds of thousands of chemicals that exist today weren’t even a twinkle in scientists eyes. If you’re no sure what it all means, I’ve got you covered! We’ll go over what it updated, what it didn’t, and what it all means for you – the consumer.

Why do we need legislative reform?
“In the United States, the average person is exposed to more than a hundred chemicals from cosmetics, soaps, and other personal care products before leaving the house in the morning. While people may assume these products are safe, their chemical ingredients are mostly untested and largely unregulated, with even known carcinogenic and endocrine disrupting chemicals still found in some formulations. What’s more, ingredient labels can be misleading, leaving even the savviest consumers in the dark about the safety of the products they use every day.” [source]
It was exactly 4 years ago that the New York Times revealed what a lot of consumers now know: “Thousands of chemicals, in billions of dollars worth of products, are being governed by regulations that haven’t been updated in decades.”
Personal care products encompass anything we use on our bodies, from toothpaste to cosmetics and shampoo or deodorant. In a 1988 Congressional hearing, it was said that “The industry’s safety apparatus was … in dire need of repair,” And after asbestos in baby powder and children’s makeup, benzene in sunscreen and deodorant, legislative reform was clearly needed.
Personal care products, including cosmetics, are regulated by the U.S. Food and Drug Administration (FDA). But they’re not treated like drugs. They don’t have to be approved by the FDA before they go on the market. So companies don’t have to prove that personal care products are safe or effective before selling them, says Dr. Alexandra White, who studies chemicals and health at the National Institute of Health (NIH). But overall, “cosmetics are one of the least regulated sets of consumer products out there,” says Dr. Ami Zota, an environmental health researcher at Columbia University. [source]
No such reforms have been passed, until now.
 the enthusiasm of our first lobbying trip to DC in 2018
the enthusiasm of our first lobbying trip to DC in 2018
The Modernization of Cosmetics Regulation Act (MoCRA) of 2022
The Modernization of Cosmetics Regulation Act incorporates significant provisions to strengthen the FDA’s ability to regulate cosmetic and personal-care products. These reforms mark the first meaningful change in cosmetics regulation since the Federal Food, Drug, and Cosmetic Act passed into law by President Franklin D. Roosevelt in 1938. While we celebrate this progress, there is more work to do.
 source: Beautycounter’s Advocacy Update, Feb 2023
source: Beautycounter’s Advocacy Update, Feb 2023
 Supporting cosmetics reform in 2020, Washington, DC
Supporting cosmetics reform in 2020, Washington, DC
Major provisions & how they help you:
- Mandatory recall authority which will enable the FDA to act when products harm consumers.
- Fragrance allergen disclosures which will require allergen disclosure to consumers.
- Registration of new Good Manufacturing Practices which will increase oversight and inspections.
- Requirements for safety substantiation to be on file which will force many brands to articulate what ingredients are safe.
- Appropriated funds and enabled NIH grants for an Interagency Task Force and green chemistry innovations.
(1) ADVERSE EVENT RECORD KEEPING AND SERIOUS ADVERSE EVENT REPORTING
“Serious adverse event” is defined as an immediate reaction of harm from a cosmetic product. Specifically, records will need to be kept on “health-related events that results in hospitalization, disability, birth defects, infection or significant disfigurement in appearance (including serious and persistent rashes, second- or third-degree burns, significant hair loss, or persistent or significant alteration of appearance), other than as intended, under conditions of use that are customary or usual.”
How does it help you?
There have been several instances of serious adverse events where the FDA previously had no recourse to act, such as when formaldehyde was found in hair straighteners and DevaCurl which both led to baldness. Because companies must report within 15 days, products are more likely to be pulled or recalled before causing widespread harm.
(2) MANDATORY RECALL AUTHORITY
In what will shock most consumers, the FDA has not been able to protect consumers with recalls until now. MoCRA grants the FDA the ability to do so.
How does it help you?
Hopefully you won’t accidentally be buying something that can knowingly give you cancer or give you permanent baldness! Companies will no longer be able to choose to leave the toxic products on the shelf. Sadly, there are so many examples from recent years when FDA needed this authority. For example, when the maker of children’s makeup chose to not voluntarily recall their products from the shelf despite finding asbestos in the talc used in the powdered makeup. This would also apply to the above hair straightener concerns as well as mercury in skin-lightening creams.
(3) COSMETIC INGREDIENT SAFETY SUBSTANTIATION
For the first time ever, brands will be held accountable to use safe ingredients. How is that defined? “The product (and ingredients) are not contrary to the use or injurious.” This means that before putting a product to market, safety substantiation will be required to be on file. Specifically, showing how an ingredient is safe, at what levels, and combined with other ingredients in the formula. This would address, for example, Formaldehyde Releasing preservatives.
How does it help you?
No longer can brands assume that an ingredient or product is safe (which has historically been the standard for almost all products in the market today). Often users, consumers, YOU are the ones who have adverse health effects from products or educate yourself on why to avoid certain ingredients. Consumers no longer need a chemistry degree to be safe. Well, that is, if FDA utilizes their power to probe into unsubstantiated ingredients or products; they may choose not to, which is often the case with underfunded government programs.
 Beautycounter’s 2018 lobbying trip to DC in support of cosmetics reform.
Beautycounter’s 2018 lobbying trip to DC in support of cosmetics reform.
Other helpful provisions:
- Mandatory registration and product ingredient listing will ensure the FDA knows by who and where products originate at the manufacturing level.
- Like most federal regulation, the Cosmetic Good Manufacturing Practices (cGMPs) now allows public input to strengthen the standards.
- If they have cause, the FDA can suspend a manufacturing facility to address serious adverse events (for example, we saw this through the pandemic in meat plants).
- Clarity for over-the-counter (OTC) drug and cosmetic jurisdiction will ensure a product manufacturers follow OTC standards (for example, higher percentages of salicylic acid or retinol are evaluated as non-OTC).
- Two of the most hotly discussed topics in personal care right now are around PFAS and Talc (which can be contaminated with asbestos). The new section addresses standardization of Talc testing, to avoid brands being able to claim non-responsibility. It also includes evaluation of PFAS safety, with potential for eliminating intentionally added PFAS depending on results. While we’d prefer to see a ban on these known hazardous ingredients, this is a step in the right direction.
- The laws already enacted defines some preemptions. Good news: States can continue to ban specific toxic chemicals, protections for overexposed communities like people of color, and transparency requirements that help address the fragrance loophole. Bad: States are prohibited from passing their own (more strict) legislation which are addressed in MoCRA. For example, it might the case that some disclosure law in California are preempted by MoCRA, which means California is less health protective in that one area. But overall this is an ideal solution that enables states to take necessary steps when appropriate.
 Beautycounter team visiting DC in 2022
Beautycounter team visiting DC in 2022
How will FDA do all this?
The spending bill that enacted MoCRA not only funds the FDA for these regulations, but also appropriates funds to other agencies to work with the FDA to make strides in this area. Considering it was reported in 2017 that the FDA only had 6 inspectors overseeing 3 million shipments of cosmetics, this seemed to be the lynchpin for having the law passed for so long. Brands won’t have to pay fees, which helps level the playing field for safety to all consumers.
The One that’s Not So Helpful: Fragrance Allergen & Ingredient Transparency
Unfortunately, the fragrance loophole was not closed with MoCRA. However, the FDA is required to create a rule for disclosure of known fragrance allergens. This has the potential to be helpful for those who have a true allergy (to nut oils, for example) or very sensitive populations. Mirroring legislation passed in California, the the Modernization of Cosmetics Regulation Act (MoCRA) does require professional salon products to have the same level of disclosure as products sold at retail. Of course, that all feels quite arbitrary with the exceptions under the fragrance loophole, but it’s a start.
Why is Fragrance so bad?
You’ll notice that “fragrance” or “parfum” or “scent” is the reason that both myself and EWG rate certain items as a higher risk. Remember, even if a brand says “natural” there is no defined standard for what that means. There’s no safety testing of an item, either. So, even if a brand has the best of intentions and thinks they’re purchasing clean rose oil from a perfumery, unless they are conducting their own safety and ingredient testing to know exactly what is in it, the perfumery could add chemicals that enhance the scent or have it last longer (like phthalates).
An example: this NIH study on chemicals coming from laundry vents found “those scented emissions that are classified as hazardous air pollutants and known or probable carcinogens” as defined by the EPA. And yet we’re putting it in our homes, on our bodies for extended periods of time. Known carcinogens on our body: pass!
It’s also the reason that over half of makeup on the market tested has PFAS, with 88% of those brands not disclosing any ingredients that would contain it… but they had “fragrance.”
Fragrance is the loophole in personal products, intended to protect company’s trade secret formulas – now it “hides” things from consumers. With California’s recent state laws we will start to see more brands disclose what is in them – either on the label or the required online database – to the benefit of the world. But, until then you have absolutely no way of knowing what is in a product if it states a fragrance of any kind, no matter the claim. If it really is just essential oils, why not just list exactly what they are and what went in.
 Gregg Renfrew, Beautycounter Founder, and Stacy Toth lobbying in DC 2022
Gregg Renfrew, Beautycounter Founder, and Stacy Toth lobbying in DC 2022
From the front lines
The bill is called the Modernization of Cosmetics Regulation Act of 2022 (MoCRA) and was included in a large omnibus spending bill that helped avert a government shutdown. While the legislation has about 65% of the reforms needed to fully protect consumers, the timing of this was critical. Had Congress failed to pass these provisions, due to a split Congress and the following Presidential election, realistically we would have had to wait another five years for reform to pass.” Read her recap: Congress Passed Major Updates to Cosmetics Safety Laws, the First Since 1938
 Christy Coleman (pro clean beauty MUA) and Stacy lobbying in DC
Christy Coleman (pro clean beauty MUA) and Stacy lobbying in DC
What is the timeline?
We can expect to start seeing these changes in 2024, as follows:
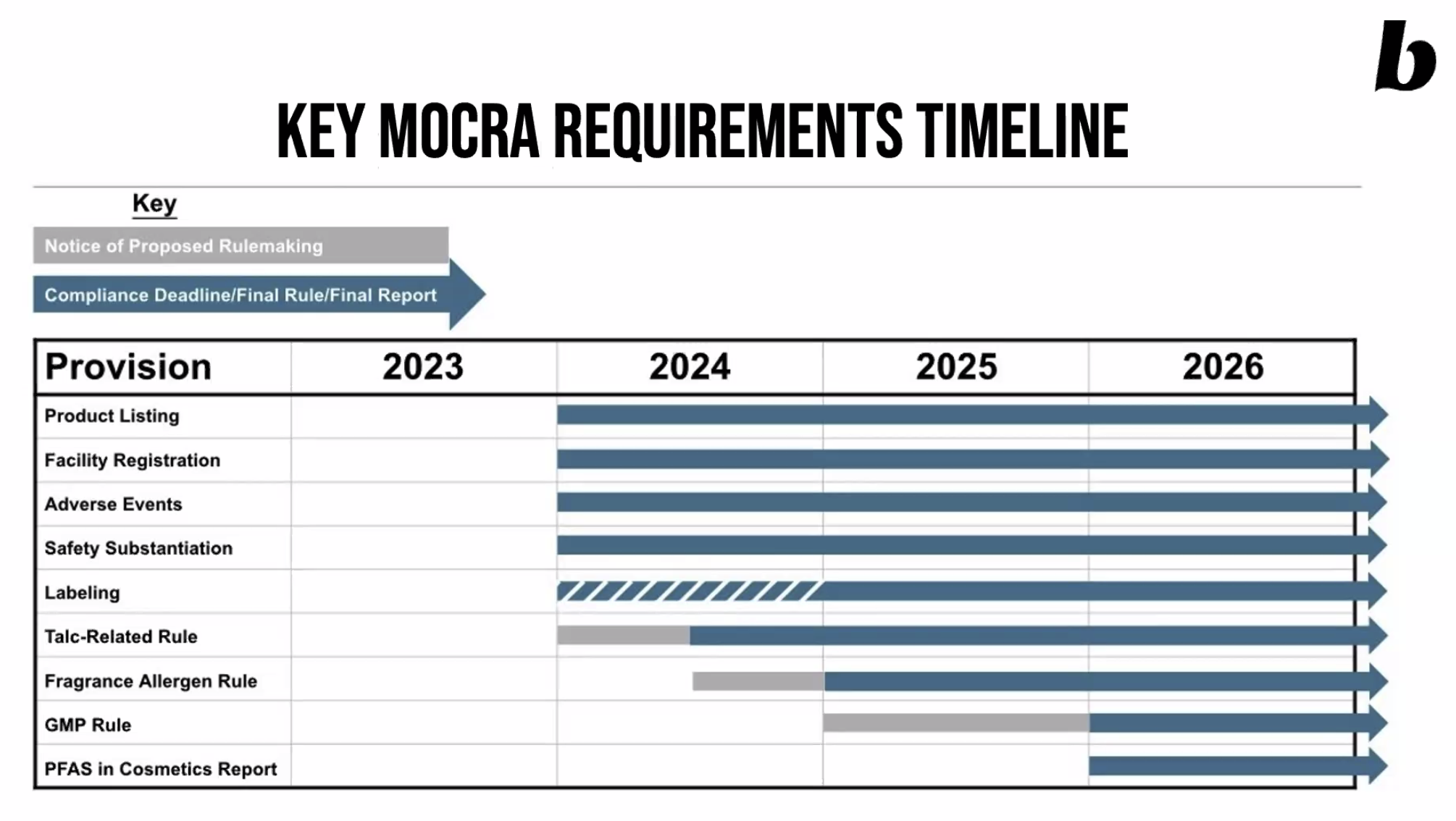 source: Beautycounter’s Advocacy Update, Feb 2023
source: Beautycounter’s Advocacy Update, Feb 2023
What are ingredients of concern?
I want to live in a world where every single person can walk into any store and the product they buy off the shelf is safe. This is an assumption a lot of Americans have about their personal care that is unfortunately not true. What ingredients should we look for?
The general classes we’re concerned about include phthalates, parabens, PFAS, and metals like lead,” as well as other problem chemicals, such as “triclosan and triclocarban… Many chemicals of concern, including phthalates, parabens, PFAS, and triclosan, are endocrine disruptors. These are compounds that can mimic or interfere with the body’s hormones. They’ve been linked to problems with the brain, development, and reproduction. Some have also been linked to a higher risk of certain cancer types. Metals like lead and mercury can cause damage to the brain. Another ingredient to look out for is formaldehyde. It is found in some hair products or created when hair products are heated. Formaldehyde exposure has been linked with cancer. Talc is also a common ingredient in cosmetics; but talc can sometimes be contaminated with asbestos, which is linked to cancer. [source]
The problem is, products do not disclose their ingredients – especially those undisclosed from the “fragrance loophole.” A full list of ingredients I personally avoid are:
- Parabens
- Phthalates
- Sulfates (SLS)
- Microplastics
- Nanoparticles
- Chemical sunscreens (oxybenzone, octinoxate, octocrylene)
- Petroleum, Petrolatum, Mineral Oil
- Butylated hydroxytoluene (BHT, or toluene)
- Butylated hydroxyanisole (BHA)
- Retinol and Retinol derivatives
- “Fragrance” or “Parfum”
What’s next?
None of this change could have happened without your support and our collective voice. When you choose to shop Beautycounter with me, it allows me to continue to advocate for change. Collectively we sent 236,000 emails, made over 16,000 calls, and held over 2,200 meetings with Members of Congress. While we didn’t get everything we wanted in the first pass, they heard us!
Additional bills are under review. Continued support will let legislators know you are looking for them to continue to make health protections in personal care a priority. The goal is for every single ingredient and formula reviewed for:
- Carcinogenicity
- Mutagenicity
- Developmental toxicity
- Reproductive toxicity
- Endocrine disruption
- Environmental Persistence and Bioaccumulation
 from our 2022 lobbying trip that helped push MoCRA forward
from our 2022 lobbying trip that helped push MoCRA forward
Some of the pending Federal legislature that will help us make progress towards this goal are:
- Safer Beauty Bill Package includes:
- Cosmetic Fragrance and Flavor Ingredient Right to Know Act
- Toxic-Free Beauty Act
- Cosmetic Safety for Communities of Color and Professional Salon Workers Act
- Cosmetic Supply Chain Transparency Act
- No PFAS in Cosmetics Act
Pending State Legislature
- Washington Non-Toxic Cosmetics Act
did not pass in 2022, Reintroduced in 2023 - Oregon Chemicals of Concern Used in Cosmetics Products Bill
- Illinois Cosmetic Product Safety Bill
- Nevada Intentionally Added PFAS Bill
- New Jersey Protecting Against Forever Chemicals (PFAS) Act
- Rhode Island Comprehensive PFAS Ban Act
- Massachusetts Act to Protect from PFAS
If you live in any of these states, contacting your officials could have a HUGE impact!
In Washington especially, we’re hopeful the reintroduced Non-Toxic Cosmetics Act can pass. If you want to support this text TOXICFREECOSMETICS to 52886
And in any US area at anytime you can text BETTERBEAUTY to 52886 for up to date communication customizable for your own representatives that will take less than 2 minutes to send! It’s good to send these several times a year as Beautycounter updates the letters to remind your lawmakers.
State laws come into effect more quickly and with less health protective features negotiated out. Just last year several state laws passed:
- California PFAS Free Cosmetics Act (2022)
- New York Mercury Out of Cosmetics Bill (2022)
I will continue to advocate on behalf of our community!
In fact, if you look carefully you can see me in this advocacy video:
References:
- Harvard.edu: Harmful, untested chemicals rife in personal care products
- FDA.gov Advises Consumers to Stop Using Certain Cosmetic Products
- NYT: Do You Know What’s in Your Cosmetics?
- NYT.com: Did a Cult Hair-Care Line Cause Thousands of Women to Lose Their Hair?
- FDA.gov: Formaldehyde in Hair Smoothing Products: What You Should Know
- FDA.gov: Mercury Poisoning Linked to Skin Products
- CleavelandClinic.org: Benzene Found in Sunscreen: Here’s What You Need To Know
- NIH.gov: Probing Personal Care Products, Look Out for Harmful Ingredients
- NIH.gov: Formaldehyde may be found in cosmetic products even when unlabelled
- BeautyMatter: MoCRA: First Significant US Cosmetics Regulation Reform Since 1938
- CEW.org: What the Passing of the Latest Reform Act Means for the Safety of Cosmetics
- CSPINET.org: Should you be concerned about the chemicals in cosmetics?
- Glossy: Welcome to a new era of cosmetics regulations
- Beautycounter’s Advocacy Update, 2023
You can keep up each week on Instagram and our newsletters.
Never want to miss a better beauty post, sale, or deal? Join my Healthy Inside & Out email list for more info on non-toxic living and safer skincare!










